Where did the Whooley Questions originate?
The Whooley Questions originated as part of the PRIME-MD diagnostic interview which was developed by Pfizer in 1993 to help primary care physicians diagnose psychiatric disorders. Pfizer's goal was to broaden/expand prescribing of Zoloft (which Pfizer started selling in 1991) outside of psychiatry. The PRIME-MD included 5 diagnostic modules, one each for depression, anxiety, alcohol use, eating and somatoform disorders. Test characteristics of the PRIME-MD were first published by Spitzer et al in 1994.
The PRIME-MD had two parts: a "Patient Questionnaire" screening instrument and a "Clinician Evaluation" follow-up interview. The "Patient Questionnaire" (Part I of the PRIME-MD) included 30 questions about symptoms of mood, anxiety, alcohol, eating and somatoform disorders. Patients were instructed to complete this questionnaire using paper and pencil. Individuals who endorsed a threshold number of symptoms for one or more of the 5 conditions were supposed to undergo a more in-depth evaluation by the provider. For mood, the two screening questions were:
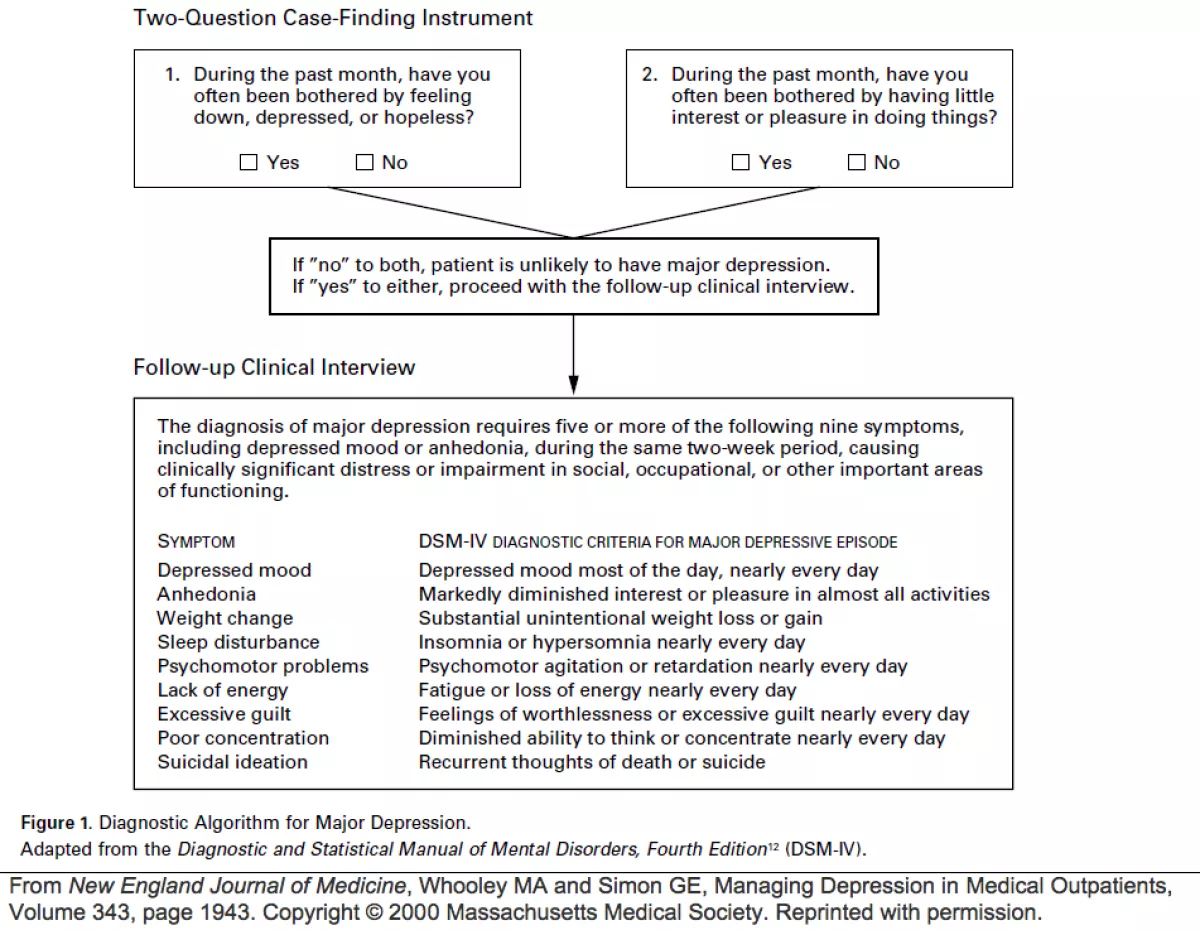
1) During the past month, have you often been bothered by little interest or pleasure in doing things? (Yes/No)
2) During the past month, have you often been bothered by feeling down, depressed or hopeless? (Yes/No)
A yes answer to one of these two mood questions triggered a more in-depth (15-question) “Clinician Evaluation” for depression. The "Clinician Evaluation" (Part II of the PRIME-MD) contained a total of approximately 60 follow-up interview questions that the primary care physician could use to establish a diagnosis of mood, anxiety, alcohol, eating, and/or somatoform disorder. If the patient did not endorse one of the two mood symptoms on the “Patient Questionnaire,” the “Clinician Evaluation” for mood disorder was not administered.
In 1995, Dr. Cynthia Mulrow and colleagues conducted a meta-analysis to evaluate the test characteristics of nine different case-finding instruments for identifying major depression in the primary care setting. In a paper published in Annals of Internal Medicine, they pointed out that the PRIME-MD Patient Questionnaire “included the fewest questions of all case-finding instruments that had been studied in primary care settings.” Based on data published by Spitzer et al in 1994, they reported that the two mood screening questions (from the PRIME-MD Patient Questionnaire) were 86% sensitive and 75% specific as compared with a subsequent telephone interview diagnosis of major depressive disorder. Mulrow and colleagues concluded, “Because the operating characteristics of [nine depression screening] instruments are similar, selection of a particular instrument should depend on issues such as feasibility, administration and scoring times, and the instruments’ ability to serve additional purposes, such as monitoring severity or response to therapy.”
How were the Whooley Questions validated?
In 1997, Dr. Mary Whooley and colleagues conducted a study evaluating the test characteristics of these two mood questions as a stand-alone screening instrument for depression. They hypothesized that endorsing one of the two cardinal symptoms, depressed mood or anhedonia, might serve as an efficient screening tool for major depressive disorder. In a cross-sectional study of 536 Veterans, Whooley et al compared the two questions with a gold standard diagnostic interview for major depressive disorder that was administered (in person) on the same day. They found that test characteristics of the two questions were similar to those of several longer instruments, such as the Center for Epidemiologic Studies Depression Scale (CES-D) and Beck Depression Inventory (BDI), that were commonly used at the time. As expected, all of the screening instruments had poor specificity (i.e. many false positives) and therefore could not be used to establish a diagnosis of depression. However, a no response to the two questions (score of 0 out of 2 possible points) was 96% sensitive, essentially ruling out depression.
Because this 2-question instrument was substantially shorter and easier to administer than any other screening tool, it was quickly adopted and recommended for routine depression screening by the US Preventive Services Task Force, the US Department of Veterans Affairs, and the UK National Institute for Health and Care Excellence (NICE). In 2000, Drs. Whooley and Simon proposed a new diagnostic algorithm for major depressive disorder:
Four years after the Whooley Questions were validated in 1997, a self-report version of the PRIME-MD "Clinician Evaluation" was introduced as the PHQ-9 (Kroenke et al, 2001), and two years after that, the first two questions of the PHQ-9 were published as the PHQ-2 (Kroenke et al, 2003). The PHQ-2 has a different time frame (last 2 weeks vs. past month), response format (multiple choice vs. yes/no), and range of scores (0 to 6 vs. 0 to 2) than the Whooley Questions. However, because of their similarities, the Whooley Questions and PHQ-2 can easily be confused with one another.